Highly diastereoselective synthesis of 3-indolylglycines via an asymmetric oxidative heterocoupling reaction of a chiral nickel(ii) complex and indoles†
Abstract
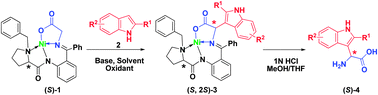
The asymmetric synthesis of 3-indolylglycine derivatives was achieved by an oxidative heterocoupling reaction. This method for the selective C-3 functionalization of unprotected

 Please wait while we load your content...
Please wait while we load your content...