Catalytic dehydrogenative Si–N coupling of pyrroles, indoles, carbazoles as well as anilines with hydrosilanes without added base†
Abstract
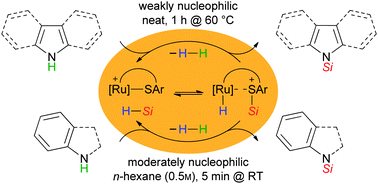
A base-free, catalytic protocol for the dehydrogenative Si–N coupling of weakly nucleophilic N–H groups of heteroarenes or aryl-substituted amines with equimolar amounts of hydrosilanes is reported. Cooperative Si–H bond activation at a Ru–S bond generates a silicon electrophile that forms a Si–N bond prior to the N–H deprotonation by an intermediate Ru–H complex, only releasing H2.

 Please wait while we load your content...
Please wait while we load your content...