A short and flexible route to tetrahydropyran-4-ones via conjugated nitrile oxidescycloaddition and oxa-Michael cyclization: a concise diastereoselective total synthesis of (±)-diospongin A†
Abstract
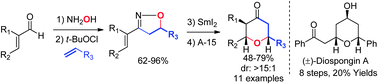
A short and flexible [3+2+1] synthetic strategy was developed for the synthesis of substituted tetrahydropyran-4-ones, featuring [3+2]-cycloaddition of α,β-unsaturated

 Please wait while we load your content...
Please wait while we load your content...