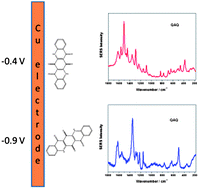
SERS detection of quinacridone quinone (QAQ), an insoluble synthetic organic pigment relevant to modern artworks, is reported here. The use of ionic liquids (BMIMCl and TBAN) as dispersing agents has allowed us to carry out electrochemical SERS experiments of QAQ in aqueous solution using a Cu electrode. No SERS spectra were obtained either from the ionic liquids (ILs) or from QAQ when silver/gold colloids were employed. The spectra of the Cu electrode in 0.1 M KCl aqueous solutions containing either BMIMCl or TBAN indicate that the corresponding cations approach the metal surface through formation of an ion pair with specifically adsorbed Cl−, but the corresponding cyclic voltammograms only show reduction and oxidation of the Cu surface. In the case of QAQ dispersed in BMIMCl and TBAN, two different SERS spectra due to QAQ species are observed between −0.4 and −1.0 V. One of the spectra agrees with the SERS of QAQ previously reported by us, using calixarenes as dispersing agents and silver colloids as SERS substrates. The other spectrum reveals a reallocation of the charge of the QAQ molecule, concomitant with a change in its relative orientation to the Cu surface. The SERS detection of QAQ is free from interferences of bands corresponding to the ILs.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...