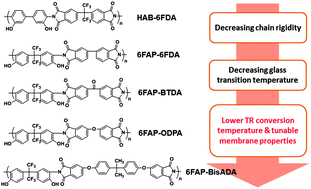
Soluble aromatic polyimides containing ortho-positioned hydroxy groups were synthesized as precursors for thermal rearrangement (TR) to polybenzoxazoles (PBOs). Fully imidized polyimides with high-molecular-weights were afforded via a ‘one-pot’ solution imidization technique (i.e., ester-acid method). The poly(hydroxyimide)s were designed to vary in their glass transition temperatures (Tg) by carefully selecting dianhydride–bisaminophenol combinations to introduce various levels of chain rigidity. TR conversion (imide-to-benzoxazole conversion) occurred in solid-state films only under inert atmosphere and over a temperature range of 300–450 °C, depending on the chemical structure (chain rigidity) of precursors. The effect of the precursor Tg on TR conversion was studied using TGA, DSC, FTIR and gel fraction measurements. The TR conversion temperature of imide-to-benzoxazole rearrangement strongly depended on the precursor Tg. Thus, for example, the feasible TR temperature was successfully reduced by ∼100 °C by lowering the precursor Tg by using a bisphenol A type dianhydride in the polymer synthesis. Gas permeation properties of representative TR systems are also reported. The TR process significantly increased gas permeabilities while maintaining good selectivities. By correlating the TR conversion degree with gas transport properties, there appears to be an optimal TR conversion degree that can maximize both gas permeability and selectivity. Systematic studies on TR polymers derived from low Tg precursors were suggested to further explore this correlation.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...