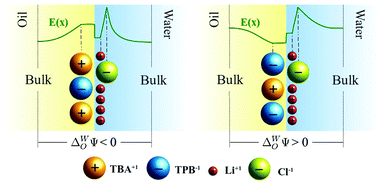
The electric field at the interface between two immiscible electrolyte solutions determines the self-assembly of synthetic and biomolecular nanoparticles at liquid interfaces and the physical properties of important biological and technological processes such as drug delivery, stability of emulsions or electro-extraction of metal ions from industrial wastewater. The classical Poisson–Boltzmann theory has been widely used to describe the corresponding ionic distribution, even though it neglects the polarization and ion correlations typical of these charged systems. Here, using Monte Carlo simulations, we provide an enhanced description of an oil–water interface in the presence of an electric field without needing any adjustable parameter, including realistic ionic sizes, ion correlations, and image charges. Our data agree with experimental measurements of excess surface tension for a wide range of electrolyte concentrations of LiCl and TBATPB (tetrabutylammonium-tetraphenylborate), contrasting with the result of the classical non-linear Poisson–Boltzmann theory. More importantly, we show that the size-asymmetry between small Li+ and large Cl− ions can significantly increase the electric field near the liquid interface, or can even reverse it locally, at high salt concentrations in the aqueous phase. These observations suggest a novel trapping/release mechanism of charged nanoparticles at oil–water interfaces in the vicinity of the point of zero charge. In the absence of an applied electric field, a difference in the mean electrostatic potential in the bulk phases of both immiscible electrolytes due to ion partitioning would also promote similar effects in synthetic and biomolecular liquid interfaces.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...