Total synthesis of (±)-paracaseolide A and initial attempts at a Lewis acid mediated dimerization of its putative biosynthetic precursor†
Abstract
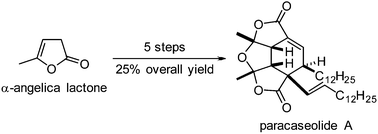
A short (5 steps) and highly efficient (25% overall yield) synthesis of paracaseolide A is described. Crucial steps are an α-iodination of a butenolide, a Suzuki coupling and a thermal Diels–Alder reaction. In attempts at Lewis acid catalyzed [4 + 2]-cycloadditions a set of novel dimerization products of the proposed biosynthetic paracaseolide A precursor were produced.

 Please wait while we load your content...
Please wait while we load your content...