Piceatannol, a better peroxyl radical scavenger than resveratrol†
Abstract
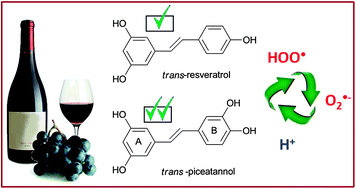
The peroxyl radical scavenging activity of piceatannol has been studied in aqueous and lipid solutions, using the density functional theory, and compared to that of its structural analogue resveratrol. The following mechanisms of reaction have been considered: hydrogen transfer (HT), single electron transfer (SET) and radical adduct formation (RAF). Piceatannol was found to be a better peroxyl scavenger than resveratrol, regardless of the polarity of the environment. In aqueous solution, at physiological pH, its higher activity is directly related to its acidity. The further reactions of the phenoxyl radical products (yielded by the reactions of ˙OOH with piceatannol and resveratrol) with the superoxide radical anion have also been investigated. It was found that in the aqueous phase, at physiological pH, resveratrol and piceatannol can be efficiently regenerated after scavenging two free radicals (˙OOH and ˙O2−). This suggests that these compounds have the ability of scavenging more than 2 radical equivalents, under such conditions.

 Please wait while we load your content...
Please wait while we load your content...