H2O2-triggered shape transformation of silver nanospheres to nanoprisms with controllable longitudinal LSPR wavelengths†
Abstract
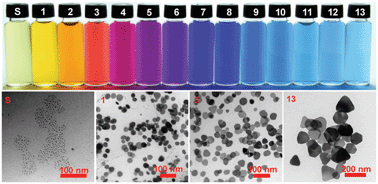
A novel approach for the synthesis of colloidal silver nanoprisms (AgNPrs) with controllable localized surface plasmon resonance (LSPR) via a chemical shape transformation of silver nanospheres (AgNSs) is presented. The shape conversion is carried out by feeding hydrogen peroxide (H2O2) solution into a starch-stabilized AgNS colloid under ambient conditions. Oxidative dissolution and the mild reducing action of H2O2 under alkaline conditions serve as the principal reactions for the shape transformation process. After addition of H2O2, the instantaneous shape transformation events can be visualized by the naked eye through the color change of the colloid. Initial concentration of AgNSs, molar ratio of H2O2 : AgNSs, H2O2 injection rate, and mixing efficiency are the key parameters for controlling the LSPR wavelengths of AgNPrs as the in-plane dipole plasmon resonance can be selectively tuned across visible and near infrared regions (i.e., 460–850 nm). The obtained AgNPrs exhibited mixed geometries e.g. hexagonal, truncated triangular, rounded-tip triangular prisms, and circular disks with average bisector lengths of 30 to 120 nm and the thickness of 10 to 20 nm. A colloid of highly concentrated AgNPrs having a final concentration up to 11 mM can be produced within 10 min.

 Please wait while we load your content...
Please wait while we load your content...