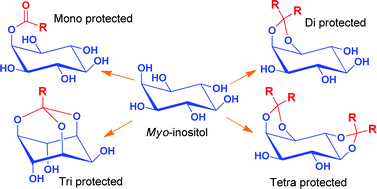
There is enormous interest in myo-inositol derivatives as they serve as precursors for the synthesis of several biologically important phosphoinositols, natural products, catalyst, supramolecular architectures etc. However the presence of six secondary hydroxyl groups of similar reactivity warrants protection of inositol hydroxyl groups for effective synthesis. Acid catalyzed protection of inositol hydroxyl groups as orthoesters or ketals are the most commonly used protecting strategy in inositol chemistry. Traditionally, homogeneous acid catalysts such as para-toluene sulfonic acid (p-TSA) or camphorsulfonic acid (CSA) are used for these transformations. While the reversible nature of these reactions necessitates the catalyst removal, aqueous work up cannot be employed for their removal as the products are water soluble. We have circumvented this problem by using H2SO4-silica as the solid supported catalyst, which can be removed by filtration, for these transformations. Treatment of myo-inositol with trialkylorthoesters in presence of H2SO4-silica under normal conditions resulted in esterification at the least reactive hydroxyl group (C2-OH) giving exclusively the corresponding 2-O-acyl-myo-inositol. By doing the reaction in a rotary evaporator under reduced pressure resulted in the formation of the corresponding orthoesters, wherein three hydroxyl groups of inositol are protected simultaneously. We could synthesize different myo-inositol orthoesters 6–10 in excellent yields by this method. Monoketaliziation of myo-inositol with one equivalent of 1,1-dimethoxycyclohexane or 2,2-dimethoxypropane in presence of H2SO4-silica under similar conditions resulted in the simultaneous protection of 1-OH and 2-OH giving the 1,2-O-cyclohexylidene-myo-inositol (11) or 1,2-O-isopropylidene-myo-inositol (12) in excellent yields. Also, diketalization of myo-inositol with 2,2-dimethoxypropane gave three diketals namely (±)-1,2:4,5-di-O-isopropylidene-myo-inositol (17), (±)-1,2:5,6-di-O-isopropylidene-myo-inositol (18) and (±)-1,2:3,4-di-O-isopropylidene-myo-inositol (19) in considerable yields. Similarly when dimethoxycyclohexane was used, the dicyclohexylidene derivatives (±)-1,2:4,5-di-O-cyclohexylidene-myo-inositol (20), (±)-1,2:5,6-di-O-cyclohexylidene-myo-inositol (21) and (±)-1,2:3,4-di-O-cyclohexylidene-myo-inositol (22), were obtained in good yield. While the yields of 1,2:3,4-di-O-alkylidene-myo-inositols are negligibly small by other known methods, interestingly, our method give these diketals in good yields and hence can be exploited synthetically. Thus by using a cheap, eco-friendly, easy-to-make and easy-to-handle H2SO4-silica as the catalyst, we could tune the conditions to make mono-protected, di-protected, tri-protected or tetra-protected inositol derivatives. These strategies can be applied for the economic synthesis of various key intermediates of inositol for various purposes.

 Please wait while we load your content...
Please wait while we load your content...