Energy and electron transfer processes in polymeric nanoparticles†
Abstract
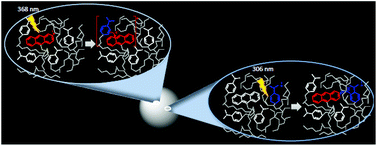
We report herein a study on photoinduced electron transfer (eT) and energy transfer (ET) processes occurring between 9-methylanthracene-acrylate (A) and N,N-dimethylaniline-acrylate (D) derivatives incorporated into polymeric nanoparticles (NP). Five types of NPs were synthesized: PAD0, PAD25, PAD75, PD25, and PD75. All NPs are composed of a crosslinked polymer matrix of methyl methacrylate and ethylene glycol dimethacrylate. In addition, PAD0, PAD25 and PAD75 contain low doping levels of A. For PAD25 and PAD75, 25% and 75% of the mole fraction of methyl methacrylate is replaced by D, respectively. PD25 and PD75 were prepared as above but without A. NPs (diameter 6–9 nm) dispersed in organic solvents were characterized based on their UV-visible absorption, emission, excitation, and excitation anisotropy spectra and time dependent absorption and emission spectroscopy techniques. The emission decay profiles of A and D were always complex. Results indicate that A senses two distinct environments in all NPs. The emission quenching of PAD0 by DMA in DCM solutions is dynamic, and it is apparent that a significant fraction of A is inaccessible to the quencher. The emission of A is efficiently quenched by the presence of D in PAD25 and PAD75. The intra-NP photoinduced eT quenching mechanism has static and dynamic components. Selective excitation of D in PAD25 and PAD75 leads to the formation of the excited state of A via a singlet–singlet ET Föster type mechanism. Results indicate that both intra-NP eT and ET processes are more efficient in PAD75 due to the reduced average D*–A separation in these NPs.

 Please wait while we load your content...
Please wait while we load your content...