Comparison of 1,4-distyrylfluorene and 1,4-distyrylbenzene analogues: synthesis, structure, electrochemistry and photophysics†
Abstract
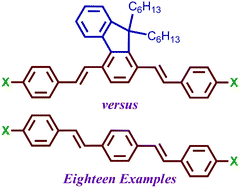
A series of nine 1,4-distyrylfluorene derivatives (2) functionalized with substituents of variable electron-donating or -accepting capabilities was synthesised. The photophysical properties of the molecules were investigated, including UV/vis absorption, photoluminescence emission, and fluorescence quantum yields. Photophysical properties of chromophores 2 were found to exhibit significant solvatochromic effects, especially in the Stokes shift and photoluminescence maxima. The electrochemical properties of series 2 were also assessed by cyclic voltammetry and differential pulse voltammetry. Results of photophysical and electrochemical analyses were further supported by DFT calculations (B3LYP/6-31G*) and single crystal X-ray diffraction on select molecules. The contributions of intermolecular π-stacking and hydrogen bonding to crystal packing are discussed. A series of nine 1,4-distyrylphenylene derivatives (3) were also synthesised and similarly characterized for comparison to photophysical and solvatochromic effects observed in series 2. Properties of similarly-substituted molecules in series 2 and 3 were compared to one another in order to assess the influence of the 1,4-fluorenylene unit.

 Please wait while we load your content...
Please wait while we load your content...