Variable coordination of redox-active TCNB in discrete and polymeric ferrocenylcopper(i) complexes: structures and spectroelectrochemical behaviour†
Abstract
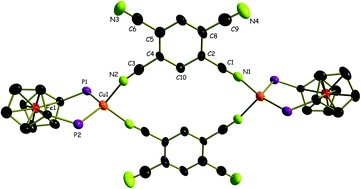
1,2,4,5-Tetracyanobenzene (TCNB) was reacted with [Cu(dppf)(CH3CN)2](BF4) and [Cu(dchpf)(CH3CN)](BF4), dppf = 1,1′-bis(diphenylphosphino)ferrocene and dchpf = 1,1′-bis(dicyclohexylphosphino)ferrocene, to produce a heterotetranuclear metallamacrocycle 1, {[Cu(dppf)(μ-TCNB)](BF4)}2, and a heterooctanuclear complex 2, [{Cu(dchpf)}4(μ4-TCNB)](BF4)4. Complex 1 is the first example of a structurally characterised discrete transition metal complex of TCNB. Upon crystallisation attempts, compound 2 formed the structurally identified coordination polymer 3, {[Cu(dchpf)(μ-TCNB)]2(BF4)2}n. Structural and spectroscopic analyses confirmed the redox-innocent behaviour of TCNB in 1, 2 and 3. However, the soluble compounds 1 and 2 could be oxidised and reduced spectroelectrochemically (UV-vis, IR, and EPR). The oxidation occurs invariably at the ferrocene sites without notable splitting of redox potentials. Reduction involves the TCNB bridging ligands to produce radical complexes. As a variably bridging acceptor component of supramolecular structures the TCNB ligand thus adopts an intermediate position between the highly electron transfer-active TCNE, TCNQ and TCNQF4 systems and the numerous redox-innocent bridges.
- This article is part of the themed collection: Coordination Programming: Science of Molecular Superstructures Towards Chemical Devices

 Please wait while we load your content...
Please wait while we load your content...