Thermolysis and solid state NMR studies of NaB3H8, NH3B3H7, and NH4B3H8†
Abstract
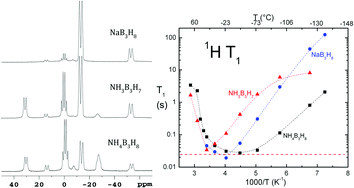
In an effort to broaden the search for high-capacity hydrogen storage materials, three triborane compounds, NaB3H8, NH3B3H7, and NH4B3H8, were studied. In addition to hydrogen, thermal decomposition also releases volatile boranes, and the relative amounts and species depend on the cations (Na+, NH4+) and the Lewis base (NH3). Static-sample hydrogen NMR is used to probe molecular motion in the three solids. In each case, the line width decreases from low temperatures to room temperature in accordance with a model of isotropic or nearly isotropic reorientations. Such motions also explain a deep minimum in the relaxation time T1. Translational diffusion never appears to be rapid on the 10−5 s time scale of NMR.
- This article is part of the themed collection: Boranes and borohydrides

 Please wait while we load your content...
Please wait while we load your content...