Role of Mn+ cations in the redox and oxygen transfer properties of BaMxAl12−xO19−δ (M = Mn, Fe, Co) nanomaterials for high temperature methane oxidation
Abstract
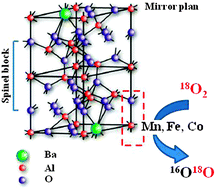
BaMxAl12−xO19−δ (M = Mn, Fe, Co, x = 1, 2) hexaaluminate nanomaterials were successfully prepared using the ARS (Activated Reactive Synthesis) process, a top-down and solvent free original synthesis route. The crystal sizes of the nanomaterials range at 24 ± 2 nm, which allows them to display high surface area (from 60 to 100 m2 g−1). The role of Mn+ cations in the redox and oxygen transfer properties of the nanomaterials was studied by H2-TPR and 18O/16O isotopic exchange, respectively. The nature of the transition metal as well as its content is observed to play a key role in the oxygen transfer properties. The catalytic properties of the nano-hexaaluminates, evaluated for methane oxidation, a reaction involving severe conditions (high temperature), resulted from multiple factors including oxygen transfer properties and transition metal valence and concentration on the surface.

 Please wait while we load your content...
Please wait while we load your content...