Tuning solid-state blue and red luminescence by the formation of solvate crystals†
Abstract
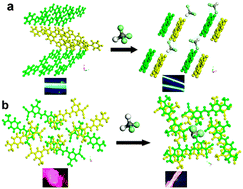
Tuning and controlling the solid-state luminescence of molecular solids play a key role in developing multi-color displays and tunable dye laser. In this work, we report the tunable blue and red luminescence by the formation of solvate crystals of 1,4-bis(5-phenyl-2-oxazolyl)benzene (POPOP) and 4-(dicyanomethylene)-2-methyl-6-(4-dimethylaminostyryl)-4H-pyran (DCM). Upon introduction of guest solvents (chloroform and dichloromethane) into the POPOP and DCM host matrices, the obtained solvate crystals exhibit an alternated stacking arrangement, interaction fashion, and crystal symmetry compared with the pristine chromophore solids. Furthermore, the solvates of POPOP (CCl3H) and DCM (CCl2H2) present changeable luminescent properties (such as one-/two-photon emissive wavelength, fluorescence lifetime and photoluminescent quantum yield) in the blue/red regions relative to the pristine POPOP and DCM. In addition, the second harmonic generation can also be obtained for the DCM (CCl2H2) due to the transformation of the centrosymmetric to a non-centrosymmetric structure from pristine DCM. Periodic density functional theoretical calculations suggest that the guest solvents do not participate in the frontier orbital distribution within the solvate crystals. Therefore, by the combination of experimental and theoretical studies on the solvate crystals, this work not only reports the supramolecular assembly of new types of host–guest photoactive systems, but also provides a detailed understanding of the electronic structures of the solid-state luminescent materials.

 Please wait while we load your content...
Please wait while we load your content...