Size-dependent oxygen storage ability of nano-sized ceria
Abstract
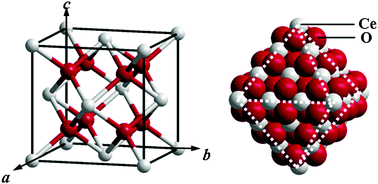
We thermodynamically studied the size-dependent oxygen storage ability of nano-sized ceria by tracing the surface Ce/O ratio of octahedral particles with different diameters, from the viewpoint of lattice Ce and O in a CeO2 crystallographic structure. The high surface Ce/O ratio with small scale particle size has more excess surface Ce4+ ions, which allows ceria to have an increasing oxygen storage ability in a crystalline lattice. For the perfect octahedron growth shape of ceria, the nonstoichiometric surfaces can produce excess Ce4+ ions, Ce4+ ions can be stabilized by bonding with lattice oxygen, leading to an enhanced oxygen storage ability of ceria. With the increasing particle size, the surface Ce/O ratio approaches to 0.5 owing to the decreased contributions of atoms located at the edges and corners. When the octahedron diameter D = 0.55 nm, the surface Ce/O ratio can reach 0.75. When D = 7.58 nm, the surface Ce/O ratio decreases down to 0.51. If D ≥ 14.61 nm, the surface Ce/O ratios are equal to 0.5. The present study deepens the insight of the size-dependent oxygen storage ability of nano-sized ceria, focusing on the size-dependent excess Ce4+ on nonstoichiometric surfaces of ceria in thermodynamics.

 Please wait while we load your content...
Please wait while we load your content...