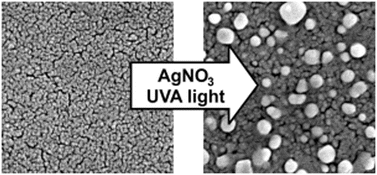
Anatase TiO2 thin-films were formed on glass by a sol–gel dip-coating method and annealed at 500 °C. Ag nanoparticles were grown on the surface of TiO2 by a photo-assisted process from AgNO3 salt using either UVC – 254 nm or UVA – 365 nm light. The size, shape and coverage of the particles were assessed by scanning electron microscopy. Changes in surface plasmon properties were investigated by UV-visible spectroscopy. A greater level of spherical Ag nanoparticles grew on TiO2 when using UVA light (365 nm); with particles 96 ± 33 nm wide on average and covering 29% of the surface. In the case of UVC light (254 nm), particles were 78 ± 14 nm wide on average and covered 13% of the surface. EXAFS measurements performed in situ of the Ag K-edge showed that the photo-assisted growth was more rapid when UVA light was used, leading to the full conversion of the AgNO3 salt layer in ≈1900 seconds. When UVC light was used, ≈50% of the salt layer was converted in ≈6100 seconds. The inhibited growth under UVC conditions was attributed to the absorption of light by the Ag nanoparticles as they formed (as opposed to the semiconductor beneath). The films also displayed reversible photochromism. The change in phase from the coloured (metallic Ag) to the bleached state (oxidized Ag) was identified using EXAFS spectroscopy. By comparing the EXAFS pattern with simulated model structures, it was shown that the transition from cubic Ag to cubic Ag2O was most likely, with an ≈70% conversion with 12 hours of white light irradiance. We believe that this is the first time the bleached form of silver in photochromic Ag–TiO2 thin-films has been identified by a direct method. In addition, we believe that this is the first case in which the photo-assisted formation of Ag–TiO2 has been monitored in situ under ambient temperature and pressure.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...