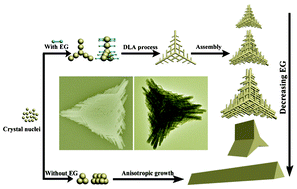
Novel dendrite-patterned triangles of Y4O(OH)9NO3:Eu3+ have been successfully prepared via a simple hydrothermal method without using any surfactant, catalyst, or template. The growth mechanism was interpreted as a modified diffusion-limited aggregation (DLA) model. Detailed contrast experiments revealed that the regulation of ethylene glycol/water ratio and pH value was essential for the formation of dendrites. The ethylene glycol served multiple functions in the formation of dendrites: to bind with Y3+ ions, change the viscosity of reaction solution, and selectively adsorb on certain faces of crystals. The as-formed precursors could transform into Y2O3:Eu3+ hierarchical dendrites after calcination, which exhibited strong red emission under ultraviolet excitation. Furthermore, the dependence of the luminescence performance on different morphologies was discussed in detail.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...