Enhancing the electrochemical performance of lithium ion batteries using mesoporous Li3V2(PO4)3/C microspheres†
Abstract
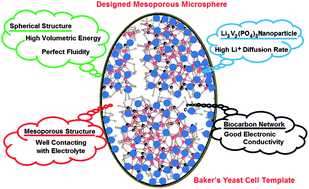
Mesoporous Li3V2(PO4)3–carbon (LVP-C) microspheres are synthesized using Baker's yeast cells as both mesoporous structure templates and amorphous carbon sources. We find that the

 Please wait while we load your content...
Please wait while we load your content...