Catalytic water oxidation at single metal sites
Abstract
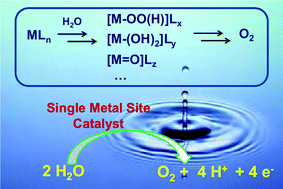
Nature utilizes solar energy to extract electrons and release protons from water, a process called photosynthetic water oxidation or oxygen evolution. This sunlight-driven reaction is vital to the planet because it directly produces dioxygen and couples with photosystem I to generate the reducing equivalents for the reduction of carbon dioxide to carbohydrates (also known as CO2 fixation). Inspired by this natural process, people are intensely interested in water splitting using sunlight to convert and store solar energy into chemical energy, which is believed to be able to ultimately solve the energy problem that we are facing. Water splitting can be separated into two half reactions, namely water oxidation and water reduction, and they can be studied individually. Catalysts are very helpful in both reactions. Recent progress in finding new highly efficient water oxidation catalysts (WOCs) has shed light on this complicated four-electron/four-proton reaction and made it possible to catalyze water oxidation using mononuclear metal complexes. This article focuses on molecular catalysts that are able to perform catalytic water oxidation at single metal sites. Different series of catalysts (or precatalysts) made of ruthenium, iridium and earth abundant elements (iron, cobalt, and manganese) that can be applied in chemical, electrochemical and photochemical (light-driven) water oxidation are summarized, and their catalytic mechanisms are discussed in detail. Finally, the future outlook and perspective to design and develop catalysts that are efficient, cheap and stable are presented.

 Please wait while we load your content...
Please wait while we load your content...