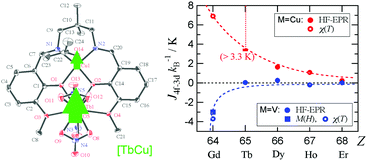
Heterometallic coordination compounds [CuII(L)(C3H6O)LnIII(NO3)3] and [VIVO(L)(C3H6O)LnIII(NO3)3] (abbreviated as LnCu and LnV, respectively; H2L = N,N′-bis(3-methoxysalicylidene)-1,3-diamino-2,2-dimethylpropane; Ln = Gd, Tb, Dy, Ho, and Er) were synthesized, and the X-ray crystallographic analysis shows that their structures are isomorphous for each series. The single-molecule magnet behavior was observed for TbCu and DyCu, and the activation energies of magnetization reversal were 42.3(4) and 11.5(10) K, respectively. The magnetic exchange couplings in LnCu and LnV were precisely evaluated by means of combined high-frequency EPR and pulsed-field magnetization studies, to give JTb–Cu/kB ≥ 3.3 K, JDy–Cu/kB = 1.63(1) K, JHo–Cu/kB = 1.09(2) K, and JEr–Cu/kB = 0.24(1) K. A monotonic decrease of ferromagnetic JLn–Cu was found in the order of the atomic number, 64Gd to 68Er. The corresponding exchange parameters in LnV are smaller than those of the Cu derivatives, and JGd–V was antiferromagnetic (−3.0 K determined from the magnetization jump). A possible mechanism for the exchange coupling and chemical trend is discussed.

 Please wait while we load your content...
Please wait while we load your content...