Electronic structure and solution behavior of a tris(N,N′-diphenylhydrazido)manganese(iv) propeller complex†
Abstract
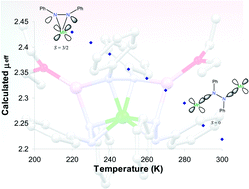
The electronic structure and magnetic properties of the manganese(IV) trihydrazide propeller complex, Li2Mn(κ2-PhN–NPh)3L2 (1, L =
- This article is part of the themed collection: New Talent: Americas

 Please wait while we load your content...
Please wait while we load your content...