Synthesis and electronic properties of a pentafluoroethyl-derivatized nickel pincer complex†
Abstract
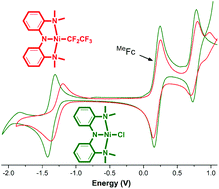
The synthesis of a bis(amino)amide nickel pincer complex bearing a perfluoroethyl
- This article is part of the themed collection: New Talent: Americas

 Please wait while we load your content...
Please wait while we load your content...