Solvent control: dinuclear versus tetranuclear complexes of a bis-tetradentate pyrimidine-based ligand†
Abstract
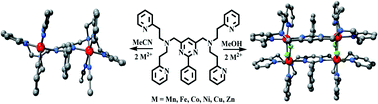
A new bis-tetradentate acyclic amine ligand LEt has been synthesized from 4,6-bis(aminomethyl)-2-phenylpyrimidine and 2-vinylpyridine. Dinuclear complexes, MnII2LEt(MeCN)(H2O)3(ClO4)4 (1), FeII2LEt(H2O)4(BF4)4 (2), CoII2LEt(H2O)3(MeCN)2(BF4)4 (3), NiII2LEt(H2O)4(BF4)4 (4), NiII2LEt(H2O)4(ClO4)4·8H2O (4′), CuII2LEt(BF4)4·MeCN (5), ZnII2LEt(BF4)2(BF4)2·½MeCN (6), were obtained from 1 : 2 reactions of LEt and the appropriate metal salts in MeCN, whereas in MeOH tetranuclear complexes, MnII4(LEt)2(OH)4(ClO4)4 (7), FeII4(LEt)2(F)4(BF4)4·![[fraction five-over-two]](https://www.rsc.org/images/entities/char_e11f.gif) H2O (8), CoII4(LEt)2(F)4(BF4)4·3H2O (9), NiII4(LEt)2(F)4(BF4)4·4H2O (10), CuII4(LEt)2(F)4(BF4)4·3H2O (11) and ZnII4(LEt)2(F)4(BF4)4 (12), result. Six complexes have been structurally characterized: in all cases each LEt is bis-tetradentate and provides a pyrimidine bridge between two metal centres. As originally anticipated, complexes 1, 4′ and 6 are dinuclear, while 9, 10 and 12 are revealed to be tetranuclear, with two M2(LEt)4+ moieties bridged by two pairs of fluoride anions. Weak to moderate antiferromagnetic coupling between the metal centres is a feature of complexes 2, 3, 4, 8, 9 and 10. The dinuclear complexes 1–6 undergo multiple, mostly irreversible, redox processes. However, the pyrimidine-based dicopper(II) complex 5 undergoes a two electron quasi-reversible reduction, CuII2 → CuI2, and this occurs at a more positive potential [Em = +0.11 V (Epc = −0.03 and Epa = +0.26 V) vs. 0.01 M AgNO3/Ag] than for either of the dicopper(II) complexes of the analogous pyrazine-based ligands.
H2O (8), CoII4(LEt)2(F)4(BF4)4·3H2O (9), NiII4(LEt)2(F)4(BF4)4·4H2O (10), CuII4(LEt)2(F)4(BF4)4·3H2O (11) and ZnII4(LEt)2(F)4(BF4)4 (12), result. Six complexes have been structurally characterized: in all cases each LEt is bis-tetradentate and provides a pyrimidine bridge between two metal centres. As originally anticipated, complexes 1, 4′ and 6 are dinuclear, while 9, 10 and 12 are revealed to be tetranuclear, with two M2(LEt)4+ moieties bridged by two pairs of fluoride anions. Weak to moderate antiferromagnetic coupling between the metal centres is a feature of complexes 2, 3, 4, 8, 9 and 10. The dinuclear complexes 1–6 undergo multiple, mostly irreversible, redox processes. However, the pyrimidine-based dicopper(II) complex 5 undergoes a two electron quasi-reversible reduction, CuII2 → CuI2, and this occurs at a more positive potential [Em = +0.11 V (Epc = −0.03 and Epa = +0.26 V) vs. 0.01 M AgNO3/Ag] than for either of the dicopper(II) complexes of the analogous pyrazine-based ligands.

 Please wait while we load your content...
Please wait while we load your content...