Synthesis and computational studies of Mg complexes supported by 2,2′:6,2′′-terpyridine ligands†
Abstract
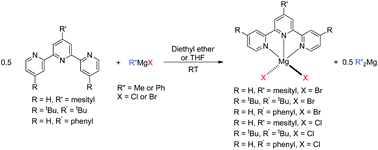
The reactions of the substituted 2,2′:6,2′′-terpyridine ligands, 4′-mesityl-2,2′:6′,2′′-terpyridine (mesitylterpy) (1a), 4,4′,4′′-tri-tert-butyl-2,2′:6′,2′′-terpyridine (tri-tButerpy) (1b) and 4′-phenyl-2,2′:6′,2′′-terpyridine (phenylterpy) (1c) with Grignard reagents were investigated. When half an equivalent of mesitylterpy or tri-tButerpy were treated with MeMgBr in diethyl ether, the only products were (R-terpy)MgBr2 (R = mesityl (5a), or tri-tBu (5b)) and Me2Mg and a similar reaction was observed in THF. Compounds 5a and 5b were characterized by X-ray crystallography. Changing the Grignard reagent to PhMgBr also generated 5a and 5b along with Ph2Mg, while the reaction between MeMgCl or PhMgCl and 1a or 1b generated (R-terpy)MgCl2 (R = mesityl (6a), or tri-tBu (6b)) and either Me2Mg or Ph2Mg, respectively. The products from reactions between phenylterpy (1c) and Grignard reagents were highly insoluble and could not be fully characterized but appeared to be the same as those from reactions with 1a and 1b. In contrast to other studies using tridentate nitrogen ligands, which formed either mixed halide alkyl species or dihalide and bis(alkyl) species depending on whether the Grignard reagent was reacted with the ligand in diethyl ether or THF, the formation of mixed halide, alkyl complexes of the type (R-terpy)MgR′X (R′ = Me or Ph; X = Cl or Br) or dialkyl species such as (R-terpy)MgR′2 (R′ = Me or Ph) was not observed here, regardless of the reaction conditions. DFT studies were performed to complement the experimental studies. The experimental results could not be accurately reproduced unless π-stacking effects associated with free terpyridine were included in the model. When these effects were included, the calculations were consistent with the experimental results which indicated that the formation of the terpy Mg dihalide species and R′2Mg (R′ = Me or Ph) is thermodynamically preferred over the formation of mixed alkyl halide Mg species. This is proposed to be due to the increased steric bulk of the terpy ligand in the coordination plane, compared with other tridentate nitrogen donors.
- This article is part of the themed collection: New Talent: Americas

 Please wait while we load your content...
Please wait while we load your content...