The ‘sticky business’ of cleaning gas-phase membrane proteins: a detergent oriented perspective†
Abstract
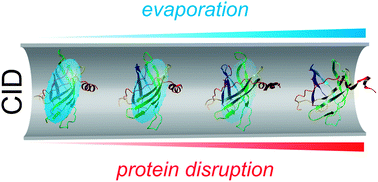
In recent years the properties of gas-phase detergent clusters have come under close scrutiny due in part to their participation in the analysis of intact membrane protein complexes by
- This article is part of the themed collection: Biophysics and biophysical chemistry in PCCP

 Please wait while we load your content...
Please wait while we load your content...