Photocatalytic reduction of nitrite to dinitrogen in aqueous suspensions of metal-loaded titanium(iv) oxide in the presence of a hole scavenger: an ensemble effect of silver and palladium co-catalysts
Abstract
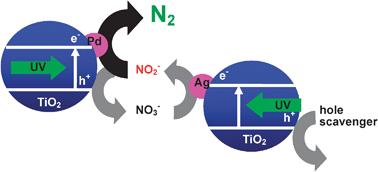
Nitrite (NO2−) was photocatalytically reduced to dinitrogen (N2) in an aqueous suspension of two kinds of titanium(IV) oxide particles loaded with palladium and silver (Pd–TiO2 and Ag–TiO2) at pH 8 under irradiation of UV light in the presence of sodium oxalate as a hole scavenger. The two metal-loaded TiO2 photocatalysts had different roles in conversion of NO2− to N2 and worked in an effective ensemble without conflict: (1) Pd–TiO2 induced photocatalytic disproportionation of NO2− to N2 and nitrate (NO3−) and (2) Ag–TiO2 selectively reduced the thus-formed NO3− back to NO2− (partially to N2) with oxalate acting as a hole scavenger. When Pd–TiO2 was used alone for NO3− reduction in the presence of sodium oxalate, Pd–TiO2 induced fruitless photocatalytic decomposition of oxalate to carbon dioxide and dihydrogen. The presence of Ag–TiO2 suppressed the fruitless decomposition of oxalate by Pd–TiO2 because Ag–TiO2 continuously provided NO2− in the reaction system using oxalate as a hole scavenger and Pd–TiO2 therefore only worked as a photocatalyst for disproportionation of NO2− to N2 and NO3− as it did when used alone.

 Please wait while we load your content...
Please wait while we load your content...