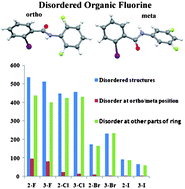
The determination of the crystal and molecular structures of a large number of compounds containing the C(sp2)-F bond has been investigated in detail in halogenated benzanilides and also in liquids, namely the fluorinated amines. It has been observed that when the fluorine atom is present in the ortho or meta position with respect to the amide functionality in benzanilides or the amino group in fluorinated amines which are liquids at room temperature, the fluorine atom exhibits positional disorder. This is associated with changes in patterns of intermolecular interactions which affect crystal packing. Furthermore, the presence of a fluorine atom on the benzanilide framework, in the presence of a heavier halogen (chloro, bromo and iodo), meta or ortho to the amide group does not eliminate the disorder associated with these molecules. In this article, we highlight the salient features present in halogenated compounds exhibiting disorder in the position of organic fluorine with concomitant changes in crystal packing. This feature is also compared with related compounds exhibiting similarity in electronic features, namely positional disorder.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...