Catalytic enantioselective construction of quaternary stereocenters by direct vinylogous Michael addition of deconjugated butenolides to nitroolefins†
Abstract
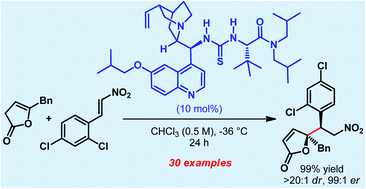
A direct vinylogous Michael reaction of γ-substituted deconjugated
- This article is part of the themed collection: 20th Anniversary of the CRSI: Celebrating Indian Chemistry in ChemComm

 Please wait while we load your content...
Please wait while we load your content...