An optimized isotopic labelling strategy of isoleucine-γ2 methyl groups for solution NMR studies of high molecular weight proteins†‡
Abstract
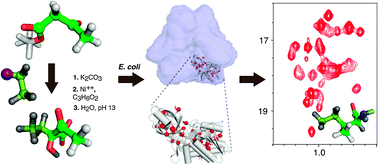
An efficient synthetic route is proposed to produce 2-hydroxy-2-ethyl-3-oxobutanoate for the specific labelling of Ile methyl-γ2groups in
- This article is part of the themed collection: Emerging Investigators 2012

 Please wait while we load your content...
Please wait while we load your content...