Hydrophobic interactions between polymer surfaces: using polystyrene as a model system†
Abstract
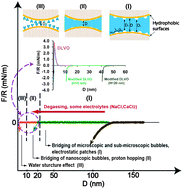
The hydrophobic interaction plays a critical role in a wide range of molecular phenomena in numerous biological and engineering systems. Using a Surface Forces Apparatus (SFA) coupled with a top-view optical microscope, we have directly measured and visualized the interactions between two polystyrene surfaces in different electrolyte solutions (i.e., NaCl, CaCl2, HCl and CH3COOH). It is evident that the long-range hydrophobic interaction measured is due to bridging of microscopic and sub-microscopic bubbles on polystyrene surfaces. The range of the hydrophobic interaction decreases with increasing the electrolyte concentration for NaCl and CaCl2, but shows no significant change for HCl and CH3COOH, which is related to the formation and stability of bubbles on hydrophobic surfaces due to the ion specificity. The range of the hydrophobic interactions was reduced to about 10–20 nm by degassing the aqueous solutions, but gradually recovered when re-exposing the degassed solution to air. Our results indicate that dissolved gasses in solutions play a crucial role in the hydrophobic interactions of
 Please wait while we load your content...
Please wait while we load your content...