Modular oxime functionalization of well-defined alkoxyamine-containing polymers
Abstract
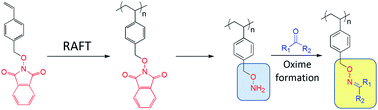
Phthalamide-protected O-(4-vinylbenzyl)-hydroxylamine was polymerized via reversible addition-fragmentation chain transfer (RAFT) polymerization with good control of the polymer molecular weight and retention of chain end functionality. The resulting polymer was deprotected by cleavage of the phthalamido protecting groups via treatment with hydrazine to reveal the latent side-chain alkoxyamine functionality (R–O–NH2). The alkoxyamine polymer scaffold was coupled with model small molecule aldehydes and ketones via highly efficient “click” oxime bond formation. The ability of the coupling reactions to be conducted at a variety of temperatures, in the presence of oxygen, and without any additional reagents makes this an attractive modular strategy for preparing well-defined polymers with high degrees of functionality.
- This article is part of the themed collection: New methods of polymer synthesis

 Please wait while we load your content...
Please wait while we load your content...