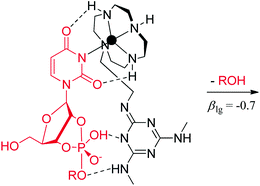
The general acid/base catalyzed cleavage of a number of alkyl esters of uridine-3′- (and -5′-)phosphate has been studied by utilizing a cleaving agent, in which the catalytic moiety (a substituted 1,3,5-triazine) is tethered to an anchoring ZnII:cyclen moiety. Around pH 7, formation of a strong ternary complex between uracil, ZnII and cyclen brings the general acid/base catalyst close to the scissile phosphodiester linkage, resulting in rate acceleration of 1–2 orders of magnitude with the uridine-3′-phosphodiesters. Curiously, no acceleration was observed with their 5′-counterparts. A βlg value of −0.7 has been determined for the general acid/base catalyzed cleavage, consistent with a proton transfer to the leaving group in the rate-limiting step.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...