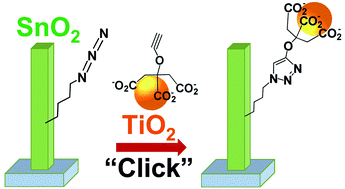
SnO2 is a promising material for photovoltaic and photocatalytic applications because it exhibits high electron mobility, its conduction band lies at a convenient energy to act as an electron acceptor, and it can be easily grown in a variety of different nanostructures including nanoparticles, nanorods, and nanosheets. However, strategies for surface functionalization of SnO2 are much less well developed than alternative oxides. Here, we demonstrate the growth and subsequent chemical functionalization of SnO2 nanorods to enable the chemically directed assembly of SnO2 nanorod–TiO2 nanoparticle heterojunctions, and we characterize the charge-transfer properties using time-resolved surface photovoltage measurements. Vertically aligned SnO2 nanorods were grown via a high-pressure chemical synthesis method. The SnO2 nanorods were square in cross-section, exposing sidewalls consisting of {110}-type crystal planes. Functionalization via photochemical grafting with butenol yielded nanorods terminated with a high density of –OH groups that were converted to azide groups. The azide groups were linked with alkyne-modified TiO2 nanoparticles via the Cu(I)-catalyzed Azide–Alkyne Cycloaddition (CuAAC) reaction, a form of “click” chemistry, thereby covalently grafting the TiO2 nanoparticles to the SnO2 nanorods. Time-resolved surface photovoltage measurements of the resulting adducts showed that the covalent bonding of TiO2 nanoparticles to the SnO2 nanorods enhances the interfacial charge transfer compared to the unmodified SnO2 nanorods, leading to an increased accumulation of holes at the surface.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...