Sulfur containing amino acids – challenge of accurate quantification
Abstract
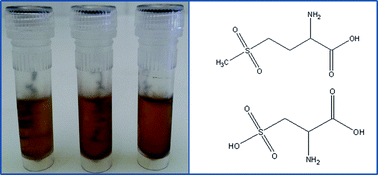
For the first time, quantitative analysis of the proteinogenic sulfur containing amino acids methionine and cysteine was performed by LC-ICP-MS. A dedicated sample preparation procedure was implemented consisting of two steps, i.e. (1) protection of the redox sensitive amino acids by controlled oxidation of methionine, cystine and cysteine to methionine sulfone and cysteic acid, respectively, and (2) subsequent protein hydrolysis. Anion exchange chromatography enabled the separation of all relevant sulfur species within 10 min. Sulfur was detected on m/z32S16O using O2 as the reaction gas. Absolute limits of detection in the pmol range were achieved for methionine sulfone and cysteic acid. The method offered the possibility of protein quantification. Absolute amounts of 2 μg of hydrolyzed protein (on column) were investigated by LC-ICP-MS. Both oxidized forms of amino acids showed excellent recoveries from lysozyme and myoglobin standards, enabling accurate quantification. A repeatability of <10% (n = 6 independently prepared samples) was found without the application of isotope dilution strategies. The limits of detection of <1 μM protein were comparable to the limits of detection achieved by spectroscopy based protein quantification assays. Moreover, the validity of the approach was shown by implementing HPLC in combination with fluorescence detection as a reference method for the quantification of proteinogenic amino acids in yeast. Both methods were in good agreement and met the theoretical value in a yeast reference material certified for the methionine content.

 Please wait while we load your content...
Please wait while we load your content...