Abstract
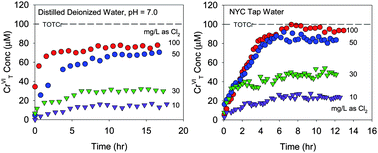
Drinking water treatment typically uses strong oxidants such as chlorine which are capable of converting CrIII to CrVI. The rates and extent of CrIII oxidation by chlorine are not well established. CrIII oxidation experiments were therefore conducted in distilled deionized water and New York City tap water dosed initially with CrIII and supplemented with sodium hypochlorite to increase free chlorine residual. Reaction progress was monitored using capillary electrophoresis which quenched reactions and allowed for quantification of CrVI. Three different forms of CrIII were used as reactants: a CrIII nitrate salt, CrIII–EDTA, and CrIII hydroxide. Rates of CrVI production for all three forms of CrIII were rapid, on the order of hours. However, oxidation rates slowed and a plateau in CrVI concentrations was reached. This resulted in less than 100% conversion of CrIII to CrVI even at relatively high chlorine doses (10 to 100 mg L−1 as Cl2). The loss of free chlorine due to a non-Cr chlorine demand, the precipitation of CrIII to Cr(OH)3(s), and the partial oxidation of CrIII to intermediate oxidation states (i.e. CrIV and CrV) were examined and eliminated as possible explanations for this behavior. Consumption of chlorine via reaction with intermediate oxidation states of Cr is therefore offered as a possible explanation for the plateau in CrVI concentrations.

 Please wait while we load your content...
Please wait while we load your content...