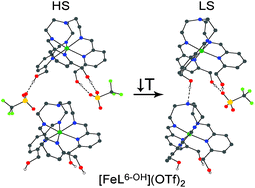
We report the syntheses, characterisations, and spin state behaviours of salts of the tripodal-ligated Fe(II) complex [FeL6-OH]X2 (L6-OH = tris{4-[(6-methanol)-2-pyridyl]-3-aza-3-butenyl}amine, X = OTf− (1), Br− (2), I− (3), BPh4− (4)). Covalent linking of the ligand arms is imperative as a high-spin bis(tridentate) complex (5) is formed when a non-tethered ethyl iminopyridine ligand (L2 = 4-[(6-methanol)-2-pyridyl]-3-aza-3-butenyl) is used. For salts 1–4, thermally-induced spin-crossover (SCO) is observed in the solid state, with dependence on anion and solvate molecules. Salts with larger anions show more complete SCO centred at higher temperatures (1 > 3 > 2); the triflate salt 1 (T1/2 = 173 K) also shows the strongest cooperativity of the compounds examined. Hydrogen bonding appears to be critical to SCO in this family of salts: limiting interactions by use of tetraphenylborate produces a high-spin complex down to 5 K. In protic solvents such as methanol, spectra of [FeL6-OH]2+ are largely unchanged over a period of three days, but dissociate when interrogated with strong field bidentate ligands. Compounds 1–3, and 5 remain high spin in solution down to 180 K, consistent with the data obtained in the solid state.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...