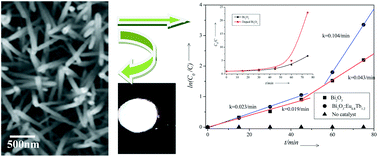
Undoped Bi2O3 and single and double doped Bi2O3 : M (where M = Tb3+ and Eu3+) nanophosphors were synthesized through a simple sonochemical process and characterized by using X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), high resolution transmission electron microscopy (HRTEM), EDS, diffuse reflectance (DRS) and photoluminescence (PL) spectrophotometry. The TEM micrographs show that resultant nanoparticles have a rod-like shape. Energy transfer was observed from host to the dopant ions. Characteristic green emissions from Tb3+ ions and red emissions from Eu3+ ions were observed. Interestingly, the Commission International de l'Eclairage (CIE) coordinates of the double doped Bi2O3 : Eu3+(0.8%) : Tb3+(1.2%) nanorods lie in the white light region of the chromaticity diagram and it has a quantum efficiency of 51%. The undoped Bi2O3 showed a band gap of 3.98 eV which is red shifted to 3.81eV in the case of double doped Bi2O3 : Eu3+(0.8%) : Tb3+(1.2%) nanorods. The photocatalytic activities of undoped nano Bi2O3 and double doped nano Bi2O3 : Eu3+(0.8%) : Tb3+(1.2%) were evaluated for the degradation of Rhodamine B under UV irradiation of 310 nm. The results showed that Bi2O3 : Eu3+(0.8%) : Tb3+(1.2%) had better photocatalytic activity compared to undoped nano Bi2O3. The evolution of CO2 was realized and these results indicated the continuous mineralization of rhodamine B during the photocatalytic process. Thus double doped Bi2O3 : Eu3+(0.8%) : Tb3+(1.2%) nanorods can be termed as a bifunctional material exhibiting both photocatalytic properties and white light emission.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...