Facile destruction of formulated chlorpyrifos through green oxidation catalysis
Abstract
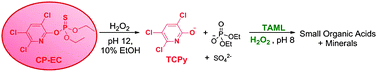
The organophosphorus (OP) insecticide, chlorpyrifos (CP, O,O-diethyl-O-3,5,6- trichloro-2-pyridyl phosphorothioate) in an emulsifiable concentrate formulation (CP-EC) is totally degraded in water by hydrogen peroxide catalytically activated by the TAML activator (1), to a combination of small aliphatic acids and minerals. CP-EC rapidly forms an oil-in-water emulsion when added to water. The CP in this emulsion is more resistant to oxidation than pure CP in aqueous solution. A one-pot, two-step process consisting of perhydrolysis followed by 1/H2O2 oxidation achieved total degradation of CP in the emulsion. In the first step, emulsified CP was hydrolyzed by H2O2 at high pH to induce the release of 3,5,6-trichloropyridin-2-ol (TCPy). The cationic surfactants CTAB or CTAC accelerated this hydrolysis. Addition of tert-butanol or ethanol also enhanced the hydrolysis rate. Xylenes serving as the solvent in CP-EC were shown to be the cause of the impeded hydrolysis. In the second step, the CP-EC hydrolysate was treated with 1/H2O2 to readily degrade the TCPy. In water, TCPy exists in the enol and not the keto form, which was found to facilitate its rapid oxidation. This degradation procedure produced a 72.5-fold reduction in toxicity (Microtox® assays) of the treated reaction mixture, compared to the untreated CP-EC emulsion. This ambient catalytic process establishes a promising line of investigation for alleviating the decades-old problem of obsolete thiophosphate pesticides.

 Please wait while we load your content...
Please wait while we load your content...