A combination of ab initio calculations with the isoelectronic principle and chemical intuition is a useful way to predict new species. Some experimentally verified examples are (1) the transition-metal hydrides, MHn (n = 4–12), (2) new members of the multiply-bonded 2nd- or 3rd-period species A![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) B, A
B, A![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) B
B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, A
C, A![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) B
B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) D or A
D or A![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) B–C
B–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) D, and A
D, and A![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) B
B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) D
D![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) E classes, the last-mentioned class including the cations N+5 and OCNCO+, (3) new members of the uranyl isoelectronic series, (4) actinyls where one of the oxygens is replaced by a 5d transition-metal (TM), (5) certain systems with noble-gas–noble-metal bonds, (6) the first argon compound HArF, (7) the cluster series of WAu12, (8) TM-centred polyazide anions, (9) covalent molecules with a central –Zn–Zn– bond, (10) tetrahedral clusters of zinc and cadmium, (11) model systems for otherwise missing multiple bonds and (12) certain endohedral A@B systems. Further series of hypothetical species were used as a tool for developing recent sets of covalent radii, for studying the endohedral intermolecular interactions in A@B systems, or for finding examples of a 32-electron rule, corresponding to the well-known 8e- and 18e-rules. For obvious reasons, much of the molecular chemistry of the superheavy elements is based on studies of hypothetical model systems.
E classes, the last-mentioned class including the cations N+5 and OCNCO+, (3) new members of the uranyl isoelectronic series, (4) actinyls where one of the oxygens is replaced by a 5d transition-metal (TM), (5) certain systems with noble-gas–noble-metal bonds, (6) the first argon compound HArF, (7) the cluster series of WAu12, (8) TM-centred polyazide anions, (9) covalent molecules with a central –Zn–Zn– bond, (10) tetrahedral clusters of zinc and cadmium, (11) model systems for otherwise missing multiple bonds and (12) certain endohedral A@B systems. Further series of hypothetical species were used as a tool for developing recent sets of covalent radii, for studying the endohedral intermolecular interactions in A@B systems, or for finding examples of a 32-electron rule, corresponding to the well-known 8e- and 18e-rules. For obvious reasons, much of the molecular chemistry of the superheavy elements is based on studies of hypothetical model systems.
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) B, A
B, A![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) B
B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, A
C, A![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) B
B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) D or A
D or A![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) B–C
B–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) D, and A
D, and A![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) B
B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) D
D![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) E classes, the last-mentioned class including the
E classes, the last-mentioned class including the 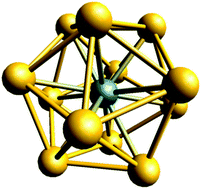

 Please wait while we load your content...
Please wait while we load your content...