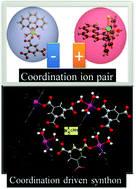
A rare supramolecular assembly involving ion pairs of coordination complexes with a host–guest relationship: synthesis, crystal structure, photoluminescence and thermal study†
Abstract
A two component new coordination complex having formula {[Mn(phen)2Cl(H2O)]+ [Mn(phen)(btc)(H2O)3]−(CH3OH)·2(H2O)} (1) [btc = 1,3,5-benzenetricarboxylate trianion; phen =

 Please wait while we load your content...
Please wait while we load your content...