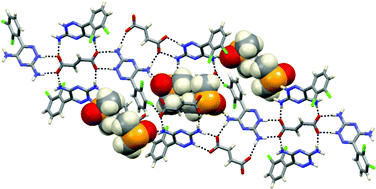
The search for materials capable of storing small molecular species is experiencing a shift from solids with permanent porosity towards organic materials capable of the uptake and release of low-molecular-weight guests. We demonstrate that a solid mixture of the pharmaceutical compound lamotrigine with a range of saturated and unsaturated 1,4-butanedicarboxylic acids, when in combination with a third molecule, can result in the formation of a family of isostructural materials involving a structurally persistent binary-host framework based on a hydrogen-bonded molecular salt of lamotrigine and the acid. A systematic study, based on mechanochemical screening, has revealed a remarkable robustness to subtle changes in the chemical functionality of the acid in that at least 12 different acids can be used in combination with lamotrigine to generate isostructural binary-host frameworks. Such robust isostructurality results in the important attribute that the shape, size and surface chemistry of the inclusion cavities can be fine-tuned by systematic variation of the substituents on the dicarboxylic acid.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...