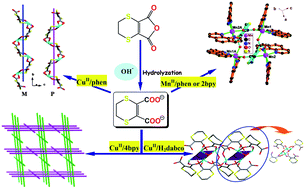
This work focuses on coordination chemistry of a heteroalicyclic dicarboxylate ligand 5,6-dihydro-1,4-dithiin-2,3-dicarboxylate (L) with transition metals in the presence of N-donor co-ligands. A series of five CuII and MnII coordination complexes with L building blocks and different chelating/bridging co-ligands have been prepared, namely [Cu(L)(phen)]n (1), [Cu(L)(4bpy)(H2O)]n (2), {[Cu2(L)2(μ3-OH)](H2dabco)0.5(H2O)}n (3), {[Mn2(L)(phen)4](H2O)2(ClO4)2}2 (4), and {[Mn2(L)(2bpy)4](2bpy)0.5(ClO4)2}2 (5) (phen = 1,10-phenanthroline, 4bpy = 4,4′-bipyridine, dabco = 1,4-diazabicyclo[2,2,2]octane, 2bpy = 2,2′-bipyridine), in which the ligand L is obtained by an in situhydrolysis reaction of 5,6-dihydro-1,4-dithiin-2,3-dicarboxylic anhydride in the presence of lithium hydroxide. Single-crystal X-ray diffraction reveals that these complexes display a variety of coordination motifs, from the discrete tetranuclear species (4 and 5) to infinite 1-D arrays (1 and 3) and 2-D → 3-D polycatenated architecture (2), which are regulated by the multiple coordination modes of L. Furthermore, the introduction of auxiliary N-heterocyclic ligands plays a critical role in extending the dimensionality of these metallosupramolecular systems via coordination and/or secondary interactions such as hydrogen bonds and aromatic stacking. The magnetic properties and thermal stability of these complexes have also been investigated and discussed in detail.

 Please wait while we load your content...
Please wait while we load your content...