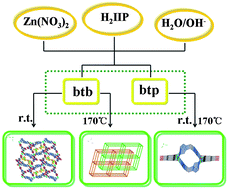
Two temperature-dependent structures of 2D and 3D Zn(II)-organic frameworks (ZOFs) with a new 5-substituted benzene-1, 3-dicarboxylic ligand, 5-iodoisophthalic acid (H2IIP), and an auxiliary flexible ligand, 1,4-bis(1,2,4-triazol-1-yl)butane (btb), with different motifs, have been investigated. Results show that when the reaction was carried out at room temperature, a undulating 2D (4,4)-network, {[Zn(IIP)(btb)]·4H2O}n (1), which further extends into a novel “soft” 3D supramolecular microporous framework with two kinds of 1D nanochannels supported by face to face π⋯π stacking interactions and C–I⋯I halogen bonds, was generated. Under hydrothermal condition at 170 °C, however, a two-fold interpenetrated 3D framework with α-Po network topology, [Zn(IIP)(btb)]n (2), would be obtained. Interestingly, both the right- and left-handed 21 helical water chains lie in one kind of the nanochannels in 1. When the auxiliary ligand was replaced by a less flexible one with a shorter spacer length, 1,3-bis(1,2,4-triazol-1-yl)propane (btp), a novel temperature-independent single-walled discrete coordination tube, {[Zn(IIP)(btp)]·2H2O}n (3), was obtained at the same two temperatures. Inside the tube is found the 21 helical water chain. Interestingly, the reversible desorption/adsorption behavior to water is significantly observed in the frameworks 1 and 3. The framework 1 falls within the category of “recoverable collapsing” and “guest-induced re-formation” frameworks. The result shows their potential application as late-model water absorbents in the field of adsorption materials. Remarkably, the first discrete single-walled Zn(II) coordination tube 3 shows high framework stability and exhibits reversible desorption/adsorption to some small guest organic molecules (methanol, ethanol and isopropanol). Furthermore, these compounds exhibit blue fluorescence in the solid state.

 Please wait while we load your content...
Please wait while we load your content...