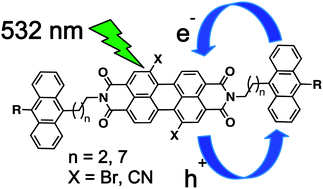
A series of donor–acceptor molecules consisting of core-brominated and -cyanated perylene-3,4:9,10-bis(dicarboximide) (PDI) structures covalently linked to two terminal pendant alkylanthracenes (A) is described. These hybrid molecules, having varying alkyl tether lengths as well as PDI electron affinities, were synthesized by condensation of a 1,7-dibromoperylene tetracarboxylic acid anhydride with the appropriate aminoalkylanthracene, followed by cyanation with CuCN. Thermal, optical, and electrochemical properties were characterized. PDI moiety photoexcitation results in pendant anthracene oxidation, generating 1(A+˙-PDI−˙-A) species. The solution dynamics of this one-electron charge separation were characterized by ultrafast transient absorption spectroscopy, and charge separation rates are found to vary with alkyl tether length. Trends in these rates are attributed to solution phase geometric variations of the PDI-A structure, reflecting the flexibility of the spacer.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...