Synthesis and structural characterization of group 6 transition metal complexes with terminal fluoromethylidyne (CF) ligands; a DFT/NBO/NRT comparison of bonding characteristics of terminal NO, CF and CH ligands
Abstract
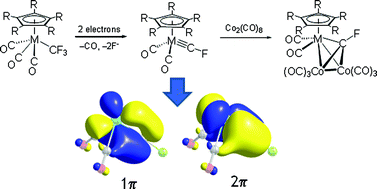
A family of group 6 transition metal complexes M(C5R5)(CO)2(CF) [M = Cr, Mo, W; R = H, Me] with terminal fluoromethylidyne ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CF subunit reacts as a triple bond to form cluster complexes containing μ3-CF
CF subunit reacts as a triple bond to form cluster complexes containing μ3-CF
- This article is part of the themed collection: Fluorine Chemistry

 Please wait while we load your content...
Please wait while we load your content...