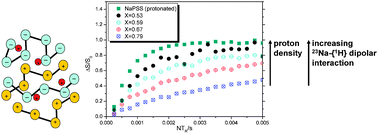
Polyelectrolyte complexes (PECs) formed by the addition of substoichiometric amounts of (poly(diallyldimethyl ammonium chloride)) (PDADMAC) solutions to sodium or lithium poly(styrene sulfonate) (Na- or Li-PSS) solution contain adjustable amounts of charge balancing Li+ or Na+ cations, which possess ionic mobility of interest for solid electrolyte applications. Very little is known regarding the local environments and the spatial distributions of these cations and their interactions with the polyelectrolyte chains in these amorphous materials. To address such issues, the present work develops a comprehensive solid state NMR strategy based on complementary high-resolution magic-angle spinning (MAS) NMR and various dipolar spectroscopic techniques. 6,7Li and 23Na chemical shifts measured on a series of PECs with general composition described by B(2x−1)PSSxPDADMA(1−x) (B = Li or Na and 0.53 ≤ x ≤ 1) reveal composition-independent local cation environments. In contrast, 7Li{6Li} spin echo double resonance (SEDOR) experiments measured on 6Li enriched materials and 7Li{1H} rotational echo double resonance (REDOR) experiments are consistent with an approximately random ion distribution. The same conclusion is suggested by 23Na{1H} REDOR measurements on the analogous sodium containing system indicating a non-segregated PEC structure. In apparent contrast to this conclusion, 23Na spin echo decay spectroscopy yields nearly constant dipolar second moments over a wide composition range. This can be explained by considering that the 23Na spin echo decays are affected by both 23Na–23Na homonuclear dipolar couplings and 23Na–1H heteronuclear dipolar interactions in the presence of strong homonuclear 1H–1H spin exchange. In protonated Na-PSS both contributions are of comparable magnitude. In the PECs the contribution from 23Na–23Na interactions decreases, while that from 23Na–1H dipolar couplings with the protons from the PDADMA chains increases with decreasing Na content, resulting in superimposed opposite dependences on the ion concentration. All results for Li and Na containing PECs point at a non-phase separated polymer network with uniform ionic sites of very similar environment. The cations can be viewed as randomly distributed and located close to the polyion sulfate groups.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...