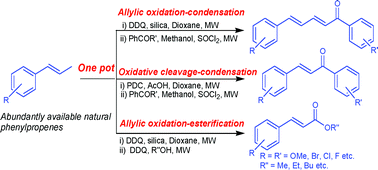
A new one-pot strategy has been developed, wherein abundantly available methoxylated phenylpropenes are directly transformed into corresponding dienones (1,5-diarylpenta-2,4-dien-1-ones) and enones (chalcones and cinnamic esters) via allylic oxidation–condensation or allylic oxidation–esterification sequences. Preliminary antimalarial activity studies of the above synthesized diaryldienones and enones against Plasmodium falciparum (Pf3D7) have shown them to be promising lead candidates for developing newer and economical antimalarial agents. In particular, two enones (12b and 13b) were found to possess comparatively better activity (IC50 = 4.0 and 3.4 μM, respectively) than licochalcone (IC50 = 4.1 μM), a well known natural antimalarial compound.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
 Please wait while we load your content...
Please wait while we load your content...