UV-induced fluorescence recovery and solubility modulation of photocaged conjugated oligomers†
Abstract
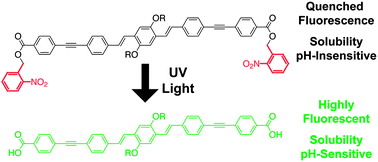
This article describes photoactivatable conjugated oligomers that show both increased fluorescence quantum yield and pH-dependant solubility upon irradiation with UV light. Sonogashira coupling between ester-substituted phenylacetylenes and alkoxy-substituted diiodo phenylene-vinylenes yielded conjugated phenylene-ethynylene/phenylene-vinylene oligomers. Oligomers with nitrobenzyl ester moieties had quenched fluorescence in polar solvents; UV irradiation restored their quantum yield of fluorescence to that of corresponding alkyl ester-substituted oligomers. These photocaged oligomers also exhibited UV-induced changes in solubility consistent with photogeneration of carboxylic acids. This approach is therefore effective at tuning the properties of conjugated organics with light after traditional synthetic operations, and has potential for use in photoactivatable fluorophores or solution-processable multilayer devices.

 Please wait while we load your content...
Please wait while we load your content...